Skype-A-Scientist
I am a regular participant in Skype-A-Scientist, a program that connects scientists with K-12 classrooms for virtual conversations.
Community Learning
Soil Analysis with 6th Graders
In Fall 2016, Ana Romano did her Community Learning Fellows research project on revising our community learning project with the Hartford Magnet Trinity College Academy 6th graders to better match their revised science standards. The new project involves soil analysis by colorimetric tests with the sixth graders and ICP-AES analysis by the instrumental analysis students. More details can be found in Ana’s final poster presentation, which she presented on campus in December 2016.

Ana Romano presents on her CLI Fellows project alongside Prof. Kovarik and our community partner, HMTCA 6th grade science teacher, Mrs. Laura Thompson.
We piloted the new experiments with Prof. Janet Morrison’s Chem 312: Instrumental Analysis students in Spring 2017. Check out their final video and poster presentations for the 6th graders!
Inorganic Water Pollutants in the Connecticut River Watershed
In the spring semester in 2014-2016, the Chem 312: Instrumental Analysis class partnered with ~100 6th graders at Hartford Magnet Trinity College Academy to analyze samples collected from around Greater Hartford. The 6th graders collected the samples and performed semi-quantitative, wet chemical determinations of chlorine, nitrate, phosphate, iron, and pH with help from the Trinity instrumental analysis students. Together the students then selected a subset of samples for further analysis by ICP-AES at Trinity. In lieu of a lab report, the Trinity students create a video report for the 6th graders summarizing our results.
More details from Spring 2014 can be found at our project blog.
Pesticide Detection in the Haw River
In Fall 2011, the Chem 431: Quantitative Analysis II students at North Carolina A&T State University partnered with ~100 5th grade students from Rankin Elementary School to look at pesticides in the Haw River outside Greensboro, NC. The A&T students analyzed Haw River water samples by SPE-GC-MS and ELISA for atrazine and simazine. They then visited the 5th graders classrooms to discuss the importance of pesticide detection, hosted them on a field trip to A&T that included a simulated ELISA experiment, and returned to Rankin for a quiz bowl style recap of the results. Photos and more details can be found at our project blog.
Small Scale for a Large Audience
As described in my 2013 Pittcon talk, I am particularly interested in outreach activities that bring micro- and nanotechnology demonstrations to broad, public audiences and K-12 students. With colleagues at IU, UNC, and Trinity College, I’ve used a number of hands-on activities to teach children and adults about tiny technology.
Simulated ELISA Diagnostic Testing
For the 2018 and 2019 TechSavvy conferences, several Trinity students, their friends, and I did a simulated ELISA experiment with middle school girls interested in STEM. We discussed the role of antibodies in our immune system and in medical testing. Using a kit from Carolina Biological, the TechSavvy participants tested 6 simulated “patient samples” for Lyme disease.
Take Water Apart: Microfluidic Electrolysis
Several Trinity students and I participated in the 2015-2017 Tech Savvy conferences for middle-school girls interested in STEM. With the middle school students, we constructed microfluidic electrolysis cells based on a 2015 Journal of Chemical Education paper. We adapted the published protocol by adding universal indicator (instead of food dye) to the water and sodium sulfate solutions. This resulted in a color change at each electrode. Using PDMS chips, 9V batteries, and platinum-plated needle electrodes, the students performed open-ended investigations of the effects of battery polarity and salt content on the electrolysis of water. Based on stoichiometry and acid-base chemistry, the students identified the anodic and cathodic sides of the cell.
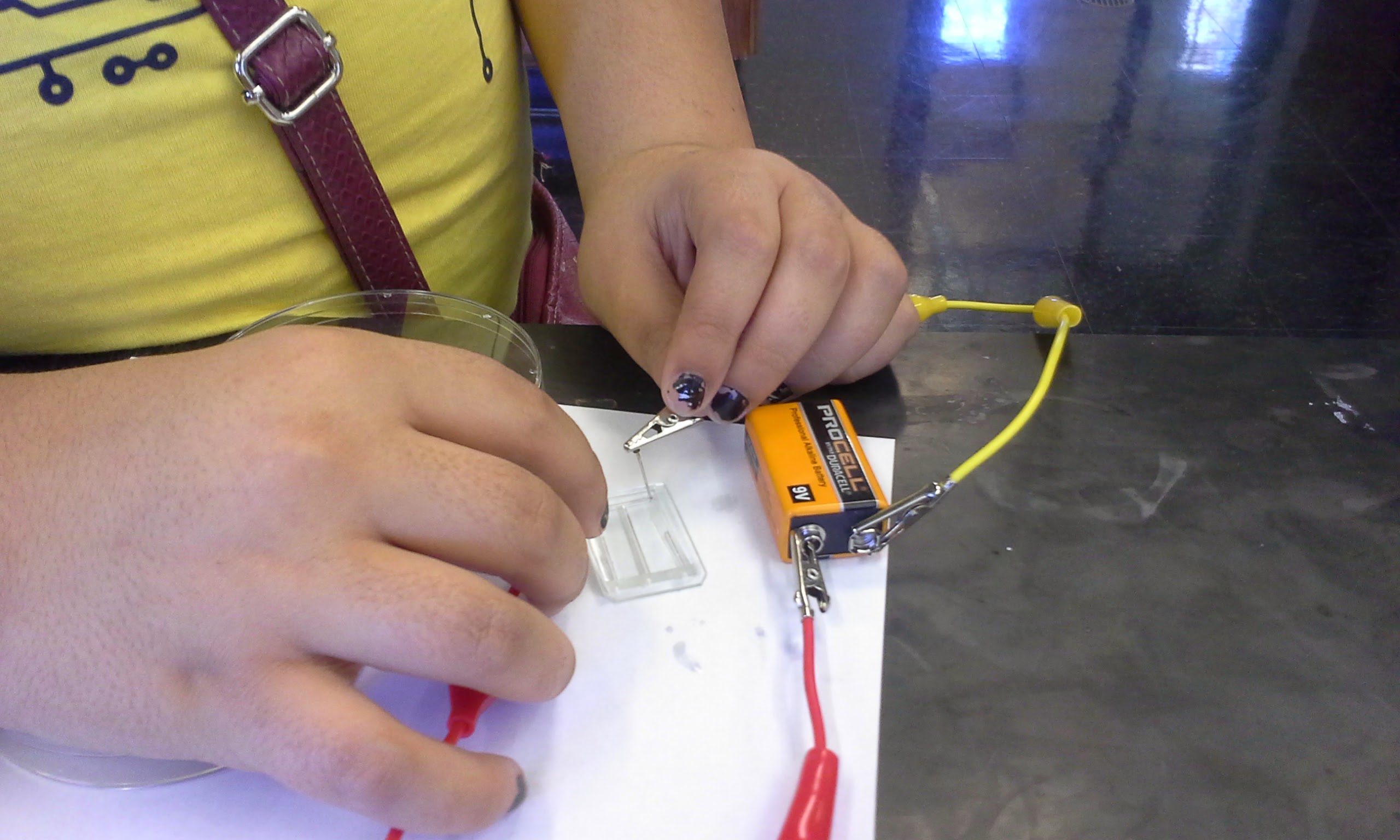
The students assembled hybrid PDMS-glass microchips, filled them with solution, inserted electrodes, and hooked up a 9V battery.

The students had the opportunity to design several experiments during the 45-minute period, allowing them to explore the effects of battery polarity, salt content and to assess reproducibility.
“Macro” rafts
The Allbritton laboratory at UNC has developed a microfabricated “raft” based technology for sorting rare cells. During my postdoc, my labmates and I constructed two “macro” sized examples of this micro-sized technology for sorting cells. Visitors to our booth raced to sort (pictures of) cells on these devices using criteria like GFP expression or active cell division. While at the UNC, my labmates and I brought this demo game to the 2012 opening of the Nature Research Center and Chemistry Day at the Museum of Natural Sciences in Raleigh, as well as the 2012 and 2013 Science Expos at UNC.
Nature Research Center Opening, Museum of Natural Sciences, Raleigh, 2012
Jell-O Microfluidics
Jell-O microchips are a fun way to demonstrate soft lithography and laminar flow to a broad audience. With my colleagues, I adapted instructions from a publication by the Lagally lab at UBC to make Jell-O chips and take-home kits for Chemistry Day at the Museum of Natural Sciences in 2012 and for the 2010 UNC Science Expo. At each event we passed out ~200 take-home kits to children and K-12 teachers to let kids design their own microfluidics at home.
Gold Nanoparticle Synthesis and Characterization
After defending my graduate work at Indiana University-Bloomington in 2009, I spent two months working with IU’s Nanoscience Center on a project with the New Tech high school Columbus Signature Academy. I helped design a problem-based learning module in which the CSA students investigated the effect of capping agent concentration on gold nanoparticle diameter. After synthesizing the particles in their school lab, the students brought their nanoparticle samples to IU for analysis by AFM and SEM.
Self-Assembled Monolayers and Chemical Patterning
To help celebrate Nanoday at the Louisville Science Center in 2009, I coordinated a booth on microcontact printing, a technique that uses a flexible stamp to apply a thin layer of material to a surface in a specific pattern. Using instructions from the Nano-CEMMS center at the University of Illinois, we helped museum visitors plate silver mirrors, stamp them with a self-assembled thiol monolayer in the pattern of their choice, and then selectively etch away the unprotected silver where the monolayer was not applied. Visitors also got to try on a cleanroom suit and learn about nanotechnology research and IU-Bloomington.





